
Later researchers painted the inside back wall with fluorescent chemicals such as zinc sulfide, to make the glow more visible. It was used in discovery of cathode rays. When they struck atoms in the glass wall, they excited their orbital electrons to higher energy levels, causing them to fluoresce.Ĭrookes tubeA Crookes tube is a rarefied tube evacuated to a pressure below 10 −6 atm. When they reached the anode end of the tube, they were traveling so fast that, although they were attracted to it, they often flew past the anode and struck the back wall of the tube. With no obstructions, these low mass particles were accelerated to high velocities by the voltage between the electrodes. By the time the tube was dark, most of the electrons could travel in straight lines from the cathode to the anode end of the tube without a collision. What was happening was that as more air was pumped from the tubes, the electrons could travel farther, on average, before they struck a gas atom. But at the anode (positive) end of the tube, the glass of the tube itself began to glow. This came to be called the cathode dark space, Faraday dark space, or Crookes dark space.Ĭrookes found that as he pumped more air out of the tubes, the Faraday dark space spread down the tube from the cathode toward the anode, until the tube was totally dark. Faraday had been the first to notice a dark space just in front of the cathode, where there was no luminescence. In the 1870s, British physicist William Crookes and others were able to evacuate rarefied tubes to a pressure below 10 −6 atm.
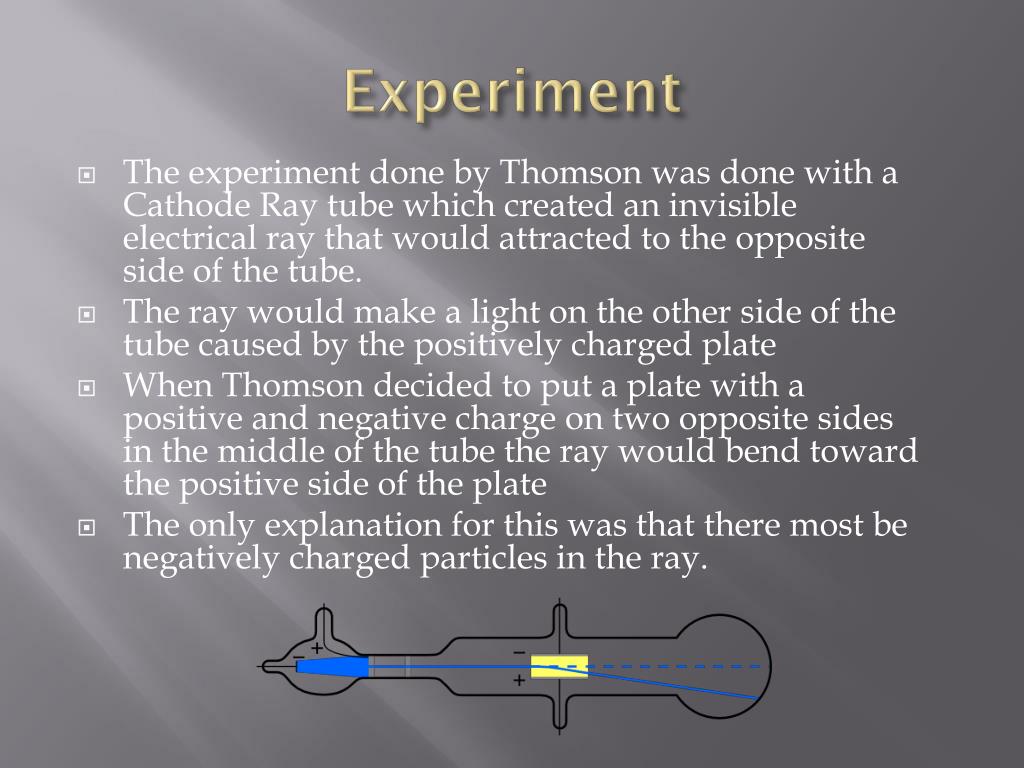
In 1838, Michael Faraday passed a current through a rarefied air-filled glass tube and noticed a strange light arc with its beginning at the cathode (negative electrode) and its end almost at the anode (positive electrode). After the electrons reach the anode, they travel through the anode wire to the power supply and back to the cathode, so cathode rays carry electric current through the tube. Researchers noticed that objects placed in the tube in front of the cathode could cast a shadow on the glowing wall, and realized that something must be traveling in straight lines from the cathode. The voltage applied between the electrodes accelerates these low mass particles to high velocities.Ĭathode rays are invisible, but their presence was first detected in early vacuum tubes when they struck the glass wall of the tube, exciting the atoms of the glass and causing them to emit light-a glow called fluorescence. They travel in straight lines through the empty tube. Since the electrons have a negative charge, they are repelled by the cathode and attracted to the anode. The increased random heat motion of the filament atoms knocks electrons out of the atoms at the surface of the filament and into the evacuated space of the tube. Modern vacuum tubes use thermionic emission, in which the cathode is made of a thin wire filament that is heated by a separate electric current passing through it. The electric field accelerated the ions and the ions released electrons when they collided with the cathode. The early cold cathode vacuum tubes, called Crookes tubes, used a high electrical potential between the anode and the cathode to ionize the residual gas in the tube. To release electrons into the tube, they must first be detached from the atoms of the cathode. The image in a classic television set is created by focused beam of electrons deflected by electric or magnetic fields in cathode ray tubes (CRTs).Ĭathode rays are so named because they are emitted by the negative electrode, or cathode, in a vacuum tube. Electrons were first discovered as the constituents of cathode rays. If an evacuated glass tube is equipped with two electrodes and a voltage is applied, the glass opposite the negative electrode is observed to glow from electrons emitted from the cathode. cathode raysStreams of electrons observed in vacuum tubesĬathode rays (also called an electron beam or an e-beam) are streams of electrons observed in vacuum tubes.crookes tubeAn early experimental electrical discharge tube, invented by English physicist William Crookes and others around 1869-1875, in which cathode rays, streams of electrons, were discovered.Thomson used the cathode ray tube to determine that atoms had small negatively charged particles inside of them, which he called "electrons."
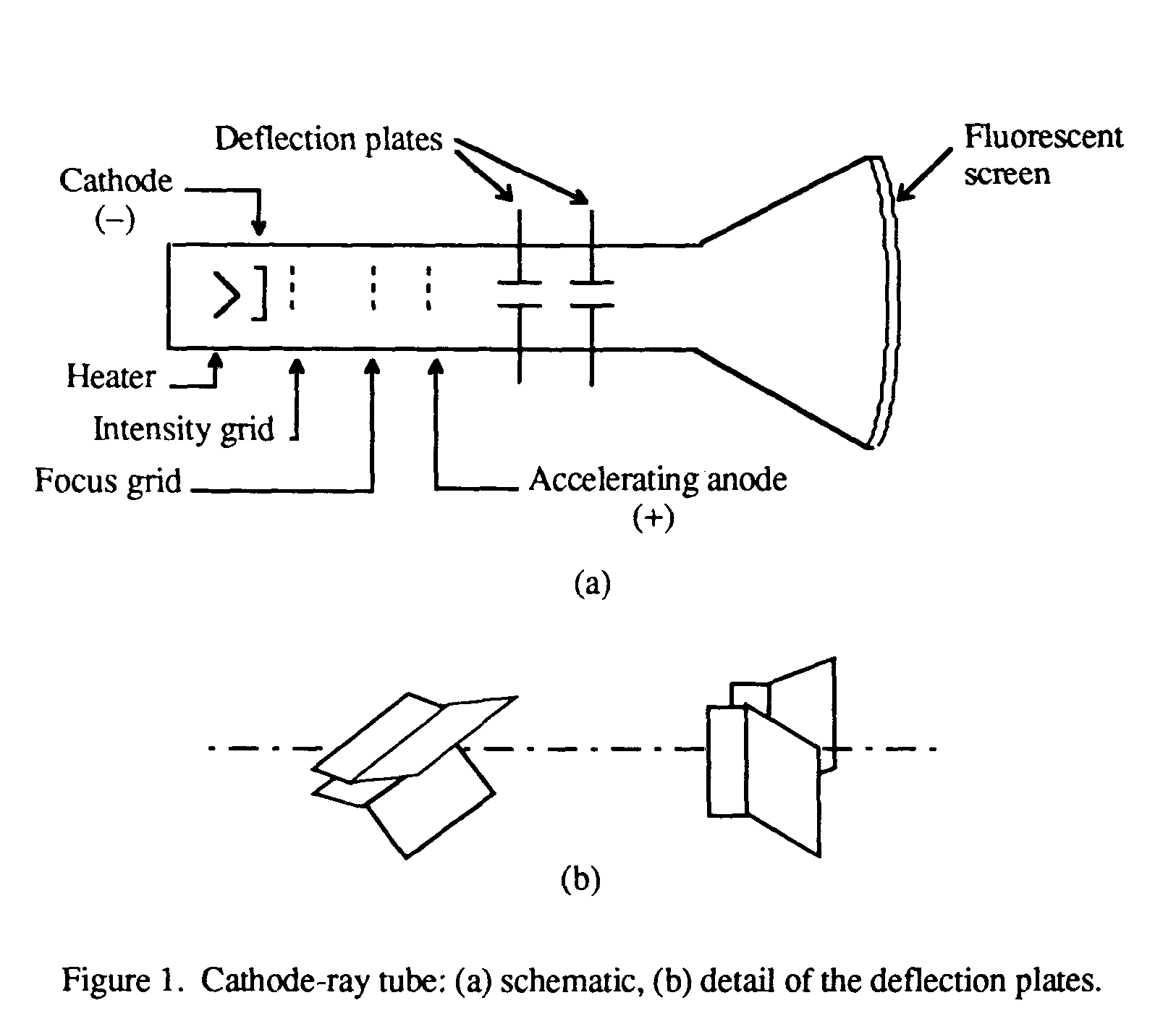

Cathode rays carry electronic currents through the tube.

Electrons accelerated to high velocities travel in straight lines through an empty cathode ray tube and strike the glass wall of the tube, causing excited atoms to fluoresce or glow.


 0 kommentar(er)
0 kommentar(er)
